How to turn a promising formulation into a scalable, commercially successful chemical product, without losing performance, consistency, or profitability along the way? This is the question R&D directors, CTOs, formulation scientists, and business strategy leaders across performance chemicals, material science, CASE, polymers, and agrochemicals ask every day.
With nearly 60% of specialty chemical companies reporting scale-up failures as their biggest commercialization challenge, organizations can no longer rely solely on internal R&D or manufacturing. The journey from concept to market requires the right blend of research expertise, process engineering, and dependable large-scale production. This gap is filled by external partners (CROs, CDMO and CMO).
Hence, understanding the roles of CROs, CDMO vs CMO has become a strategic priority. Companies across the chemical value chain, from specialty chemical manufacturers and agrochemical formulators to polymer additive developers and performance material innovators—increasingly rely on external partners.
This brings a fundamental question: Which partner model is right for your stage of development—a CRO, CDMO vs CMO
Knowing the difference between CDMO vs CMO vs CRO helps companies choose the right partner and avoid delays, quality inconsistencies, or costly scale-up setbacks.
The Evolving Needs of the Chemical Industry
Chemical companies today face a landscape defined by rapid innovation cycles, sustainability pressures, and the need for high-performance, cost-efficient products. Whether it’s a next-generation agrochemical, a high-performing polymer additive, or a greener material solution, the journey to commercial manufacturing is more complex than ever.
It requires seamless integration across:
- Research and formulation development
- Process engineering and optimization
- Scale-up and pilot production
- High-quality commercial manufacturing
And no single internal team can do it all—at least not without enormous cost and operational complexity.
This is why choosing the right external partner like Novopor matters. A contract research organization (CRO), a contract manufacturing organization (CMO), or a contract development and manufacturing organization (CDMO) will play very different roles in your innovation and growth journey.
Understanding CROs, CMOs, and CDMOs in the Chemical Manufacturing
The roles of CROs, CMOs, and CDMOs in the chemicals industry revolve around R&D capability, process engineering strength, and manufacturing scalability.
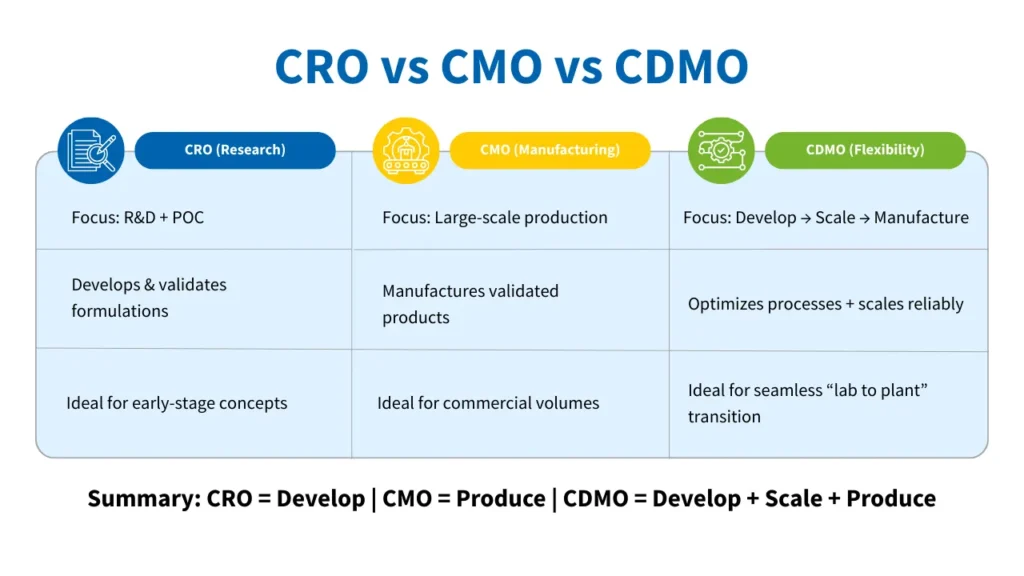
CRO – Contract Research Organization
A CRO (contract research organization) in chemicals helps companies advance ideas, validate chemistry, and refine formulations. Their focus lies in research, development, and application testing, making them essential for organizations exploring new chemistries, adjusting formulations for performance, or developing new materials for modern applications.
Chemical CRO support includes:
- Early-stage formulation and synthesis development
- Application testing (e.g., adhesion, thermal stability, rheology, agro efficacy)
- Material characterization and analytics
- Performance benchmarking against market alternatives
- Stability testing and feasibility studies
- Optimization of under-development products
CROs excel when the goal is understanding what could work and how it performs in real-world applications.
CMO – Contract Manufacturing Organization
A CMO (Contract Manufacturing Organization) focuses on large-scale production, ensuring that validated formulations or chemicals can be manufactured safely, consistently, and cost-effectively.
Their role begins once the chemistry is stable, the process is optimized and scaled up and ready to move from pilot-scale batches to commercial volumes.
Typical CMO capabilities include:
- Full-scale batch or continuous manufacturing
- Toll production
- Automation-driven process control
- Quality, safety, and environmental compliance
- Packaging, logistics, and supply chain support
- Reproducible production at scale
Choosing a CMO is ideal when the formulation is mature and the priority is vo
consistency, output reliability, and operational efficiency.
CDMO – Contract Development and Manufacturing Organization
A CDMO (contract development and manufacturing organization) integrates the strengths of both CROs and CMOs. They support the entire lifecycle—from R&D and process development to pilot-scale validation and commercial manufacturing—creating a unified pathway from concept to commercial delivery.
CDMOs typically offer:
- R&D and formulation development
- Process engineering, scale-up, and optimization
- DOE studies, yield enhancement, and cost reduction
- Pilot production for chemistry validation
- Tech transfer and manufacturing integration
- Large-scale production under quality and EHS frameworks
- Flexibility and scalability across development and manufacturing needs
- Single-roof availability of end-to-end development services managed by the same team, ensuring optimal and efficient knowledge transfer across different stages
A strong CDMO partnership reduces risk, shortens development timelines, improves manufacturability, and ensures that product performance is consistent across all stages.
How to Identify Which Partner You Need
Choosing between a CRO, CMO, or CDMO is ultimately a strategic decision that depends on your product’s maturity, the challenges you’re facing, and the capabilities you want to outsource.
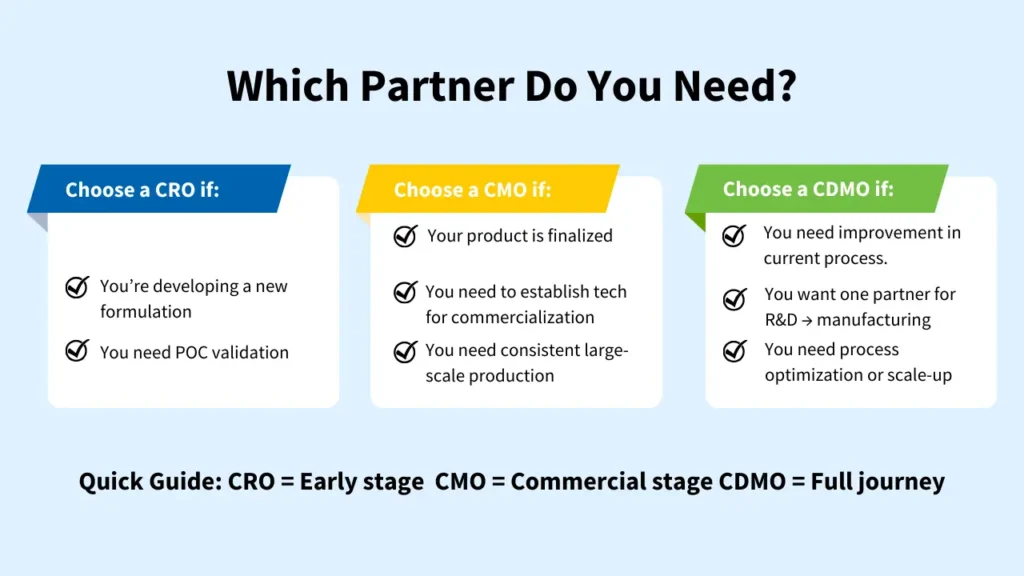
| Partner | Best For | Choose When |
| CRO | Early R&D, POC, and formulation development | • You’re exploring or validating a new material/formulation • Need application testing or analytical insights • Multiple iterations/experiments are still required |
| CMO | Commercial-scale manufacturing | • Formulation is finalized / Technology is proven developed for manufacturing. • You need consistent, repeatable large-scale production • Want cost-efficient manufacturing without new CapEx |
| CDMO | End-to-end development + scale-up + manufacturing | • You want one partner from R&D to production • Scale-up requires optimization or engineering support • Chemistry is complex and seamless tech transfer is critical |
Final Thoughts
In a manufacturing environment, choosing between a CRO, CDMO vs CMO is not just a procurement decision; it is a strategic partnership choice that can define your product’s success trajectory.
If your goal is speed, integration, and long-term collaboration, a CDMO often delivers the best blend of agility, accountability, and scalability. For companies seeking targeted expertise, CROs and CMOs still play vital, specialized roles.
As industry moves toward holistic partnerships and faster commercialization cycles, one thing is clear: the future belongs to integrated, innovation-driven CDMOs.
Looking for the right development, CDMO and manufacturing partner for your next breakthrough? Connect with Novopor, with the right experience and technicality we collaborate in your journey from concept to commercialization.
Frequently Asked Questions
Yes, especially long-term—CDMOs like Novopor optimize processes, reduce rework, and shorten timelines, resulting in lower overall development and commercialization costs.
Yes, but managing multiple vendors often leads to delays and misalignment—many biotechs now consolidate with CDMOs such as Novopor to streamline development and manufacturing.
A CDMO reduces handoff risks, accelerates time-to-market, lowers lifecycle costs, and offers single-point accountability—key advantages Novopor delivers through integrated execution.
Your choice depends on your stage: early research favors a CRO, commercial-scale production needs a CMO, and end-to-end speed and continuity are best achieved with a CDMO like Novopor.
A CRO manages research and clinical trials, a CMO focuses purely on manufacturing, while a CDMO combines development and manufacturing into one integrated, end-to-end partnership.
Look for regulatory track record, technical depth, scalability, transparent communication, and strategic advisory strength—areas where Novopor stands out.




